FAQ
Frequently Asked Questions
Safety and effectiveness proven through clinical trials of Seodong Medical Nuriai-5800
Eyes are the most precious things in the human body, and the eyeballs are protruding out, so there are many risk factors.
so Use of safety and validated productsYou must.
The eyelids are the softest of the human skin, the eyeballs should not give strong pressure or impact,
Eyeball to protect the eyes Mucus (protective film)Of untested temperatures
With hot heat It gives great damage to eyes.
so Safety, validated products.
Treatment of dry eye syndrome Medical devices approved Medical Vibrator Items
NURIEYE-5800 of Seodong Medical Food and Drug Safety Division
Clinical trials are conducted at designated clinical laboratories according to the Clinical data
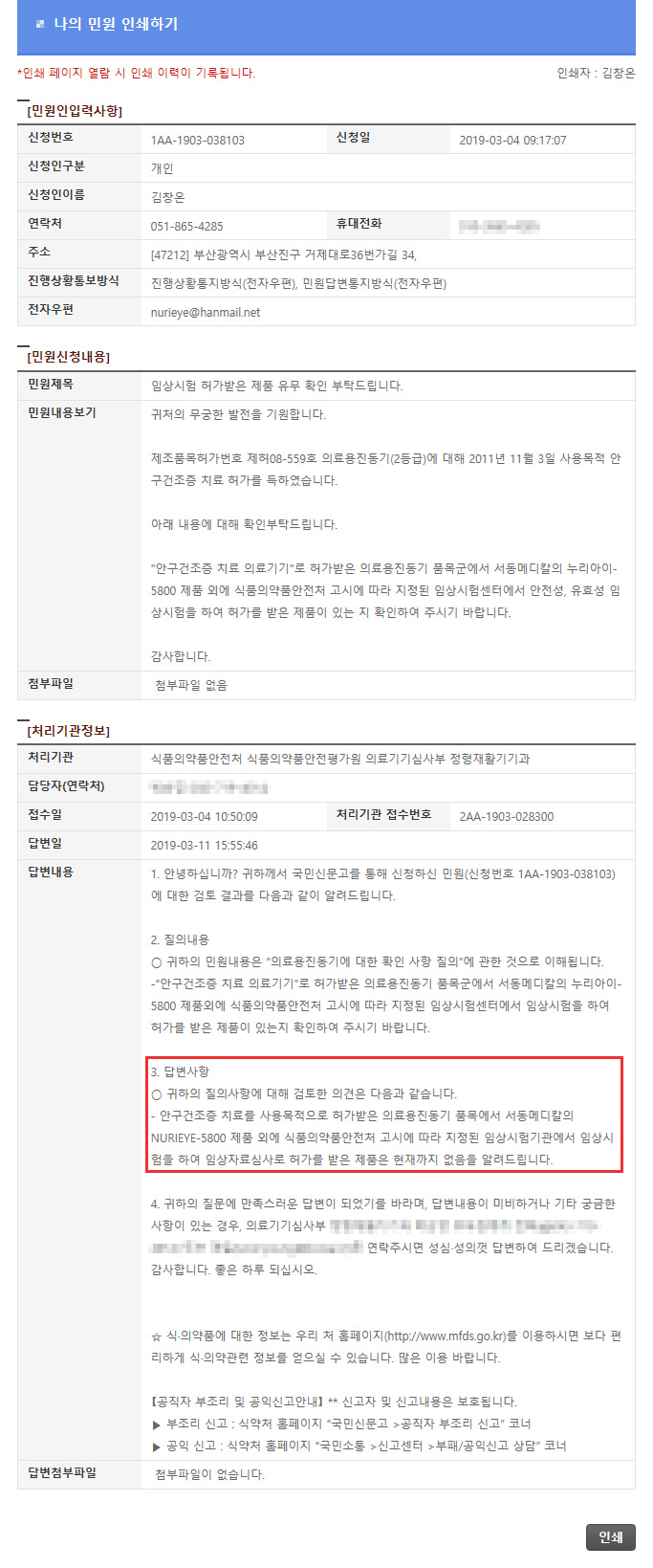
There are currently no products licensed for review.
2019.03.11 (agency response number 2AA-1903-028300)
▼ Safety and efficacy clinical trial data for the treatment of dry eye syndrome. ▼

Our eyes blink once every three seconds, and due to excessive use for 3 to 16 hours a day, they are exposed to all kinds of pollution from the environment.
In particular, due to stress caused by the use of electronic devices, fatigue accumulates in the nerves, blood vessels, and cellular tissue in the muscles around the eyes, causing the muscles to become tight and hardened, preventing tears and blood from circulating, leading to dry eyes and poor vision.
As you age, your tear ducts become clogged, making tear circulation difficult, your eyes feel stiff when you wake up, and in severe cases, the area under your eyes becomes crushed. You suffer from dry eye syndrome.
From now on, massage the area around your eyes with Nuriai every morning and evening to relieve the skin and muscles.
Nerve, blood vessels, and tears circulating smoothly Dry eye syndrome is treated.
NURIEYE-5800 product hardens the skin muscles around the eyes and blocks nerves, blood vessels, tear glands, and tear ducts.
That circulation is not good Vibration massage, pneumatic massage, warm massageIt gently relaxes the skin muscles around the eyes to improve circulation of nerves, blood vessels, and tears. The warm heat melts dust and foreign substances on the eyeballs and fatty secretions hardened in the meibomian glands inside the eyelids and helps circulation of tears. Dry eye syndrome is treated with clean tears..
Mucus on the surface of the eye is one of the tear layers that form the protective film of the eye.
The mucus is a mixture of water and oil in the tear layer, creating a protective tear film.
– Source: https://www.msdmanuals.com/ko-kr (MSD Manual) “Eye Protection Function”
originally Lift your eyelids once every 3 to 5 seconds. We need to move, but modern people use a lot of electronic devices, smartphones, etc., and driving has become more popular. Excessive strain on the eyes, once every 7 to 10 seconds Blinking eyes can cause the muscles around the eyes to harden.
When the muscles around the eyes harden, the communication of nerves, blood vessels, and tears does not go smoothly. If the circulation of tears is poor, fatty oil hardens in the meibomian glands under the eyelids and the eyes become cloudy and stiff. If the eyes are left untreated, the ducts through which tears go. These clogged tears should wash away fine dust and foreign substances and circulate, but the tear ducts are blocked, causing foreign substances and tears to accumulate, and tears that have nowhere to go end up flowing out of the eyes when the wind blows or the sunlight is strong.
Also, When fatty oil is dripping under your eyes and your vision becomes blurred You may have a problem with eye care.
It is said that the reason your eyes are blurry and your vision is blurred is because fatty oil gets stuck in the duct where tears enter.
Also, if dry eye syndrome worsens Causes keratoconjunctivitis, cataracts, eye and systemic fatigue, and even headachescan do If you leave it as it is, your eyes will be bad.

All NURIEYE-5800 series products are medical devices for treating dry eye syndrome.
Clinical studies have shown that intraocular pressure has also decreased.

NURIEYE-5800 is being tested at two university general hospital clinical trial centers in accordance with the Ministry of Food and Drug Safety notification. Safety and validityCompleted the clinical trial for the Ministry of Food and Drug Safety “Medical device for dry eye syndrome”.
Seodong Medical Nuuriai-5800 Recognized for treatment purpose by the Ministry of Health and Welfare's health insurance act non-reimbursement list notice!
Seodong Medicalin NURIEYE - 5800New Medical Technology
“Thermal massage therapy for dry eye syndrome”After being certified, I applied for and received health insurance through the Ministry of Health and Welfare, and the Health Insurance Act Non-Benefit List Notice (Ministry of Health and Welfare Notice No. 2016-104) was as follows.
Article 7 Chapter Physical Therapy “Massage therapy for dry eye syndrome” (MZ013)
* Standard non-treatment fee: 7,820 won
NURIEYE-5800 productIt has been recognized as a new medical technology evaluation and notified by the Ministry of Health and Welfare as non-reimbursable by health insurance, and has been providing treatment at eye hospitals since July 2016.
The result of examination by the Health Insurance Evaluation and Evaluation Service The treatment purpose of Nuriai-5800 was confirmed by Seodong Medical.I was recognized.

Seodong Medical Nuri Eye-5800 new medical technology “thermal massage therapy for dry eye syndrome” certified!
NURIEYE-5800 treats dry eye syndrome.Safety and effectiveness were proven through clinical trials in accordance with the government's notice and approved through the Ministry of Food and Drug Safety. “Medical device for dry eye syndrome”.
after Direct from Seodong Medical NURIEYE-5800’s dry eye treatment technology was awarded to the Korea Health and Medical Research Institute. March 2014, 3 Application and application for new medical technologyReceived
(New medical technology “Heat massage therapy for dry eye syndrome” is a technology certified by NURIEYE-5800!)
▼ Receipt for application of new medical technology directly to Korea Health & Medical Research Institute at Seodong Medical



In accordance with the regulations on the operation of the New Medical Technology Evaluation Committee (Ministry of Health and Welfare Regulations Article 61 2014.04.24), Nuriai-5800 is [Treatment of eye dryness with eye massage] (Registration number 2014-032) New medical technology is approved in accordance with the procedures of [Article 3 of the Rules on New Medical Technology Evaluation]. Safety and effectiveness evaluationBy 2014 year 11 month 3 day Ministry of Health and Welfare (Article 2014 -198) Notice Has been.
(Technical name - warm massage therapy for treating dry eye syndrome)
The warm massage therapy for the treatment of dry eye syndrome improves mybarbital function in dry eye syndrome patients Relieve symptoms of dry eye syndrome and treat.
▼ New medical technology applied in Seodong Medical 2014 year 11 month 3 day Ministry of Health and Welfare notification
It was announced as “Thermal massage therapy for dry eye syndrome”▼

A paper on the treatment of NURIEYE-5800 dry eye syndrome was published and published in the international academic journal SCI Journal and BJO (British Journal of Ophthalmology)!
International Journal SCI Journal British Journal of Ophthalmology (BJO) received an invitation to The professor who conducted the clinical trial presented the paperI did.
NURIEYE-5800 proves safety and effectiveness to treat dry eye syndromeIt was announced at an academic conference (November 2013) that Published in the academic journal BJO (British Journal of Ophthmology) in January 2014I did.






▼ BJO (British Journal of Ophthalmology)







NURIEYE-5800 was clinically tested in accordance with the Ministry of Food and Drug Safety notification.
NURIEYE - 5800은 Clinical trials were conducted at two university general hospitals in accordance with the Ministry of Food and Drug Safety notice on April 2010, 4.To the Ministry of Food and Drug Safety to proceed with “Medical device clinical trial plan approved” I applied.

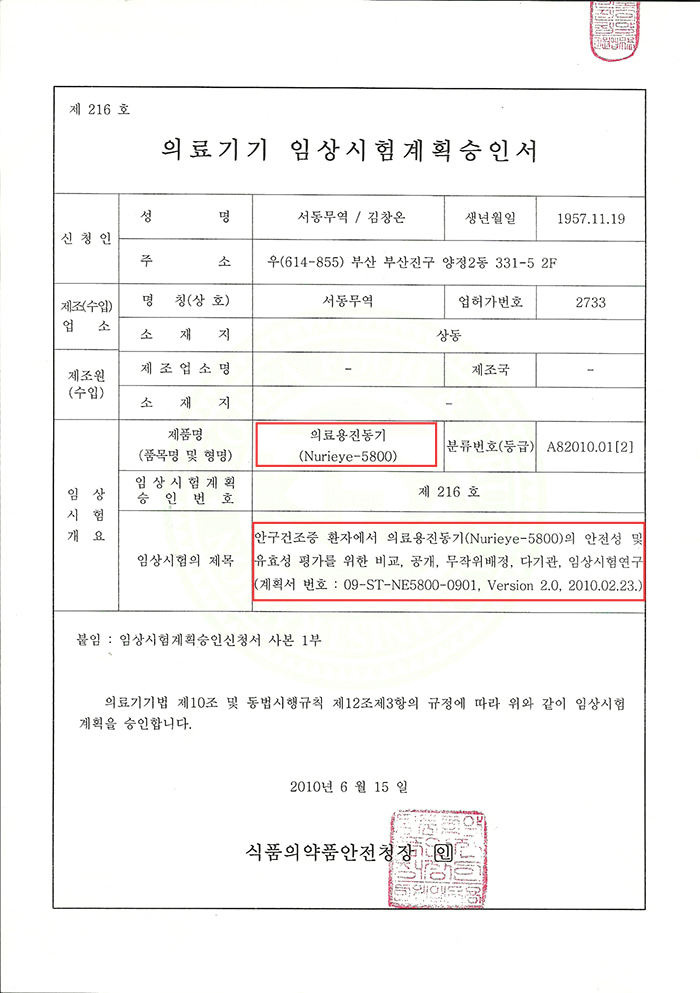
▼ We were notified of clinical trial approval through two hospitals: Pusan National University Hospital and Inje University Busan Paik Hospital. ▼
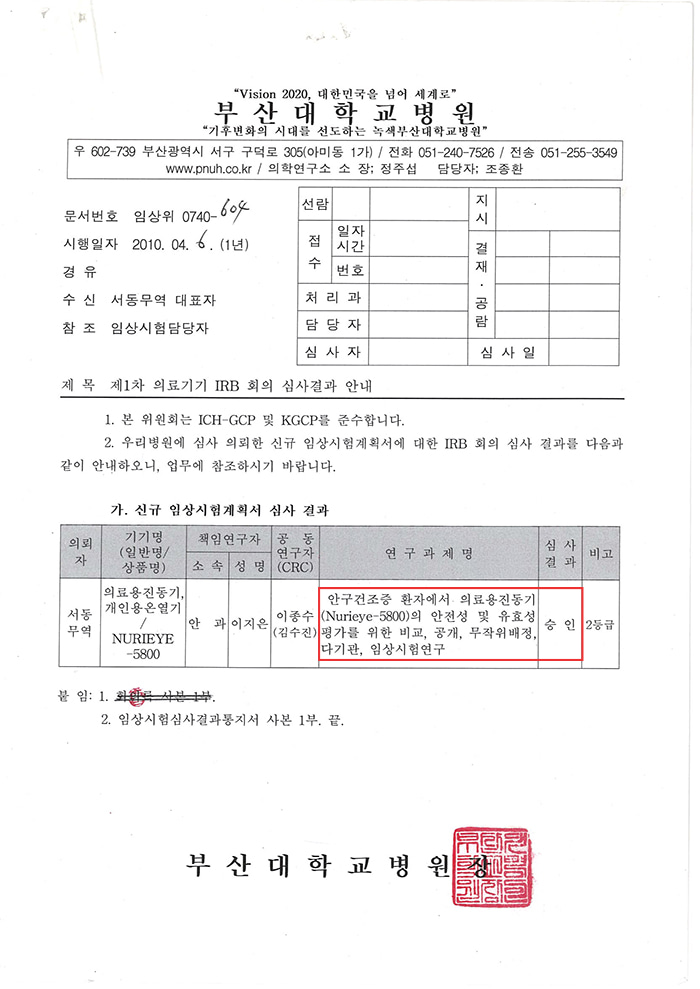
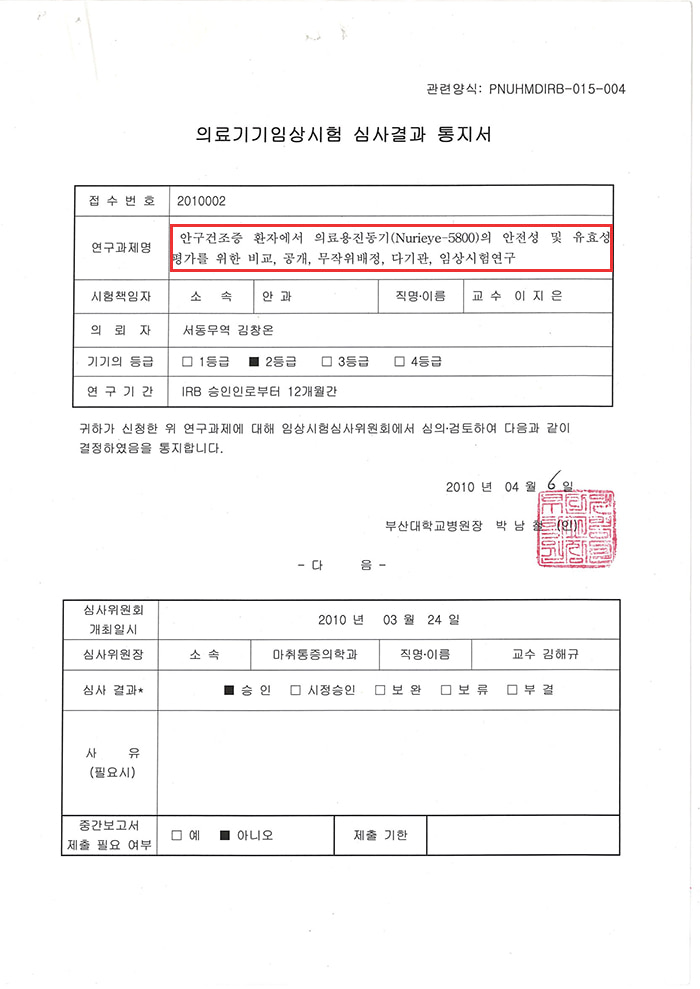
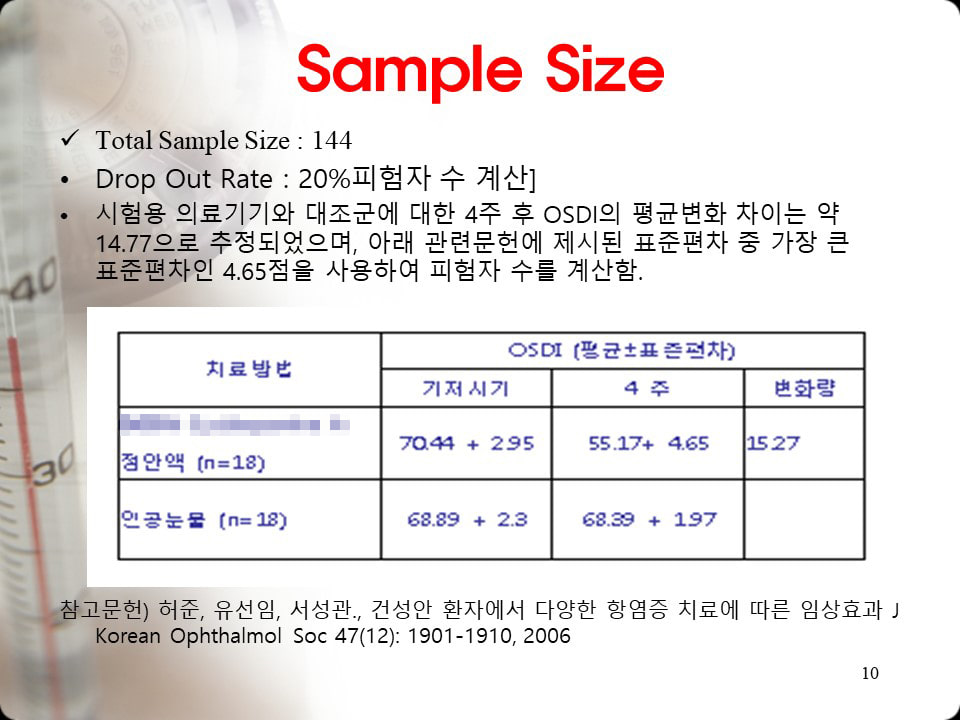
▼ Clinical trials were conducted on 144 patients with dry eye syndrome according to government statistics. ▼
The clinical trial below is compared to control group 1 (artificial tear fluid). The superiority of the experimental medical device (NURIEYE-5800), Compared to control group 2 (dry eye medication: Restasis) Demonstrate the non-inferiority of the test medical device (NURIEYE-5800)To do so.
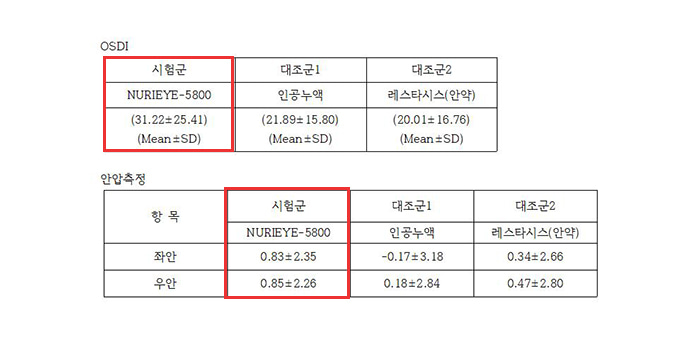
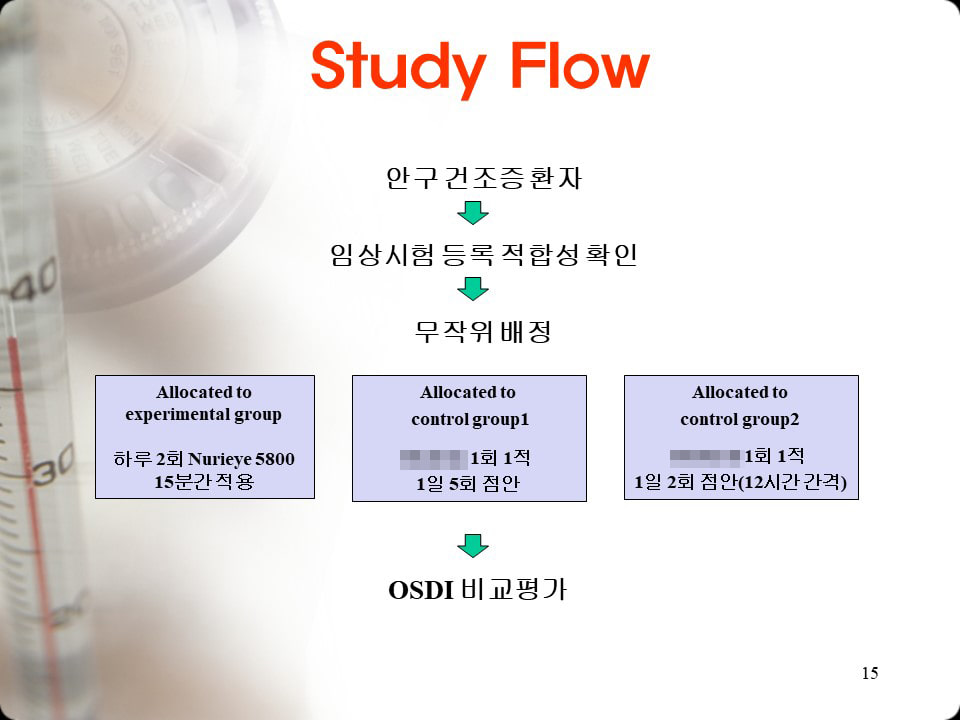
144 patients with dry eye syndromeA clinical trial was conducted at Pusan National University Hospital from November 2010, 11 to May 8, 2011 and Inje University Busan Paik Hospital from November 5, 6 to April 2010, 11, as follows.
The subject Clinical trial on safety and effectiveness using Nurieye twice a day for 1 minutes per person for 4 weeks.


Some patients with dry eye symptoms improved within a week, and as a result of a 4-week clinical trial, Dry eye syndrome treatmentHas been.
▼ NURIEYE has verified the safety and effectiveness clinical trials for dry eye treatment. ▼



















▼ We are disclosing the safety and efficacy clinical trial review (results) for NURIEYE-5800. ▼





